European Authorised Representative (EAR) which usually called "EC Representative" (EC REP). It refers to a natural person or legal person clearly specified by a manufacturer located outside the European Economic Zone EEA (including EU and EFTA). And fully assume the responsibility of product safety and consistency requested by the law within the EEA region or the European Communist Party.

Companies that are located outside the EU but selling goods in the EU must have authorized representatives since July 2021. The responsibilities of EC REP can be summarized as follows:
EC REP authorized non-EU companies to use the European address of the them on the product packaging (This is obliged)
EC REP holding technical documents, test reports and other related compliance documents
EC REP communicates with the national authority (such as the market supervision department) in the name of non-EU company
Entrusted by the manufacturer, register product in the EU, apply for free sales certificates (CFS/FSC) issued by the EU
The customs authorities have the right to confiscate and destroy entry goods if non-EU companies conduct sales activities in the EU without authorized representative addresses.
As an EC representative, we (AAMedTech GmbH) can perform the specific responsibilities required by the EU's relevant laws to perform the specific responsibilities required by the manufacturer and provide product CE certification on behalf of the EU manufacturer.
CE certification is the EU's product security certification. All medical devices entering the EU market must conduct medical device CE certification. The CE instructions that medical devices need to be met have now been upgraded to regulations, namely:
Medical Device Regulation
According to the EU's classification standards for medical device products, Class I (sterilization/measurement/reusable use), IIa, IIb, and III medical device manufacturers must use the CE marking when putting products on the market, and must pass through the certification of notified bodies, which should meets MDR (EU) 2017/745; In the Class I, in addition to sterilization/measurement/reusable products, it can obtain CE marking certification by declaration of conformity.
In Vitro Diagnostic Regulation
According to the EU's classification standards for in vitro diagnostic medical device products, Class A (required for sterilization), Class B, C, and D -type IVD medical device manufacturers must use the CE marking when putting products on the market, and must pass through the certification of notified bodies, which should complies with IVDR (EU) 2017/746; Class A non-sterilized IVD products can obtain CE marking certification by declaration of conformity.
System certification is the relevant management system certification formulated by the ISO International Standardization Organization. Through the system certification, it can intuitively reflect the level of management levels of the enterprise's quality, production environment control, and occupational health of employees, so as to achieve the pass standards for management requirements for modern enterprises. It should be said that the good operation of the management system is an inevitable guarantee for enterprises to provide good products and services.
We can undertake the counseling and certification of the following system:





The following lists some of our representative cases in the field of EC REP

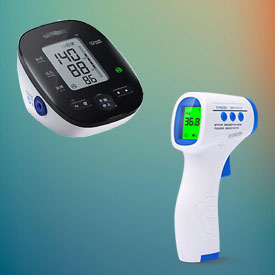

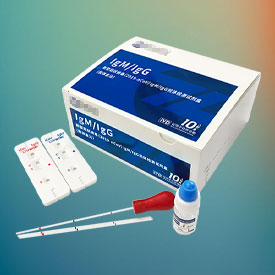
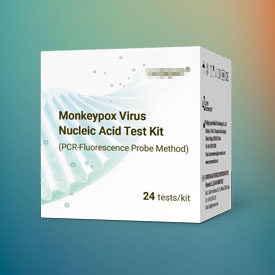








You let us know whenever you want us to update anything or think something can be optimised.
The procedures can be completed within a week normally.
The same product can only have one EC representative. If you sell a variety of products, you may need to specify multiple representatives. For example, if the products you sell are divided into "toys" categories and "electronic products" category, you may need to specify a representative for each category.
The "EC representative" is a specific responsibility related to the obligation stipulated by the manufacturer to fulfill its obligations. "EU Importers" put equipment and equipment from third countries to put in the European Union market. "Distributor" refers to any natural or legal person who supplies device and equipment in the market except manufacturers and importers in the market. The scope of duties of the three is obviously different. Secondly, the same product of each manufacturer allows only one EC representative, but there are multiple importers and sellers (distributors).
CE certification is the European qualification identification. Most of the products sold in the European Economic Zone (EEA) of European Economic Zone (EEA) need to print the "CE" marking. This marking represents the product manufacturer or service provider ensure that the product meets the corresponding European alliance instructions and has completed the corresponding evaluation procedures. Related EU instructions include medical devices, toys safety, machinery and other instructions. At present, about 25 instructions are required to print the "CE" marking.
cannot. Since Brexit, product sales to Britain need to have UK Responsible Person (like the EC representative) and pass the UKCA certification (medical devices need to complete MHRA registration). If you have relevant needs, you can also contact us to get related services.
Welcome to leave a message in the form below to get in touch with us, we will reply to you as soon as possible.